Message from Management
Long ago in 1888, the year following the establishment of the first Japanese Pharmacopoeia, we started our business as a manufacturer of essential drugs listed in Japanese Pharmacopoeia.Since then, based on our solid management policy, we have continuously striven to contribute to the betterment of healthcare and enhance people’s health and welfare through our enthusiastic business activities, covering R&D, manufacturing and marketing.
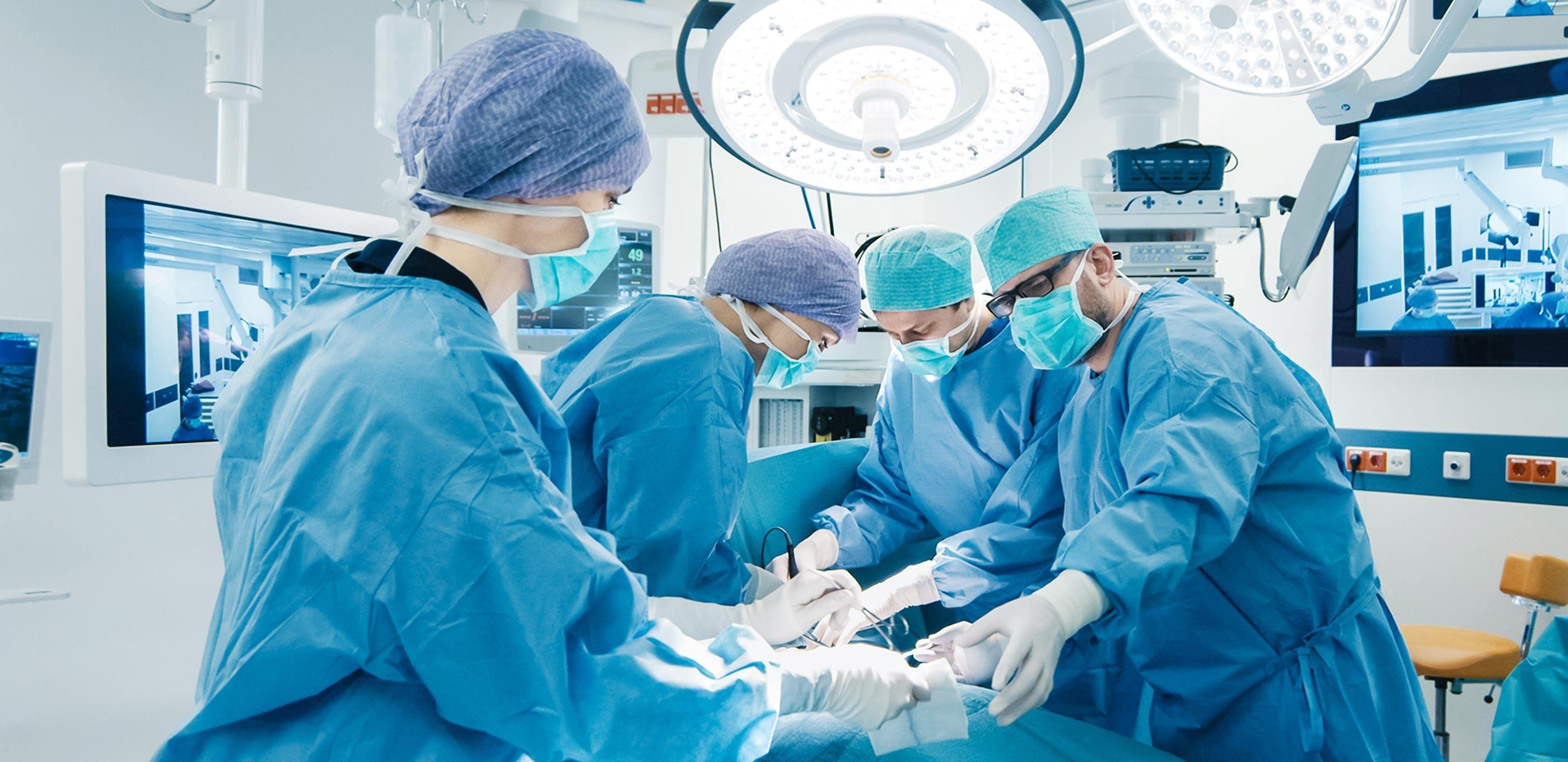
Aiming for a more advanced Specialty Pharma in the perioperative area
We are proactively engaged in R&D and sales promotional activities for perioperative drugs. Our emphasis is placed on improving the quality of life(QOL) of patients who undergo medical operations by helping to ease their pain caused by invasive treatment and pain associated with disease.

Business Activities
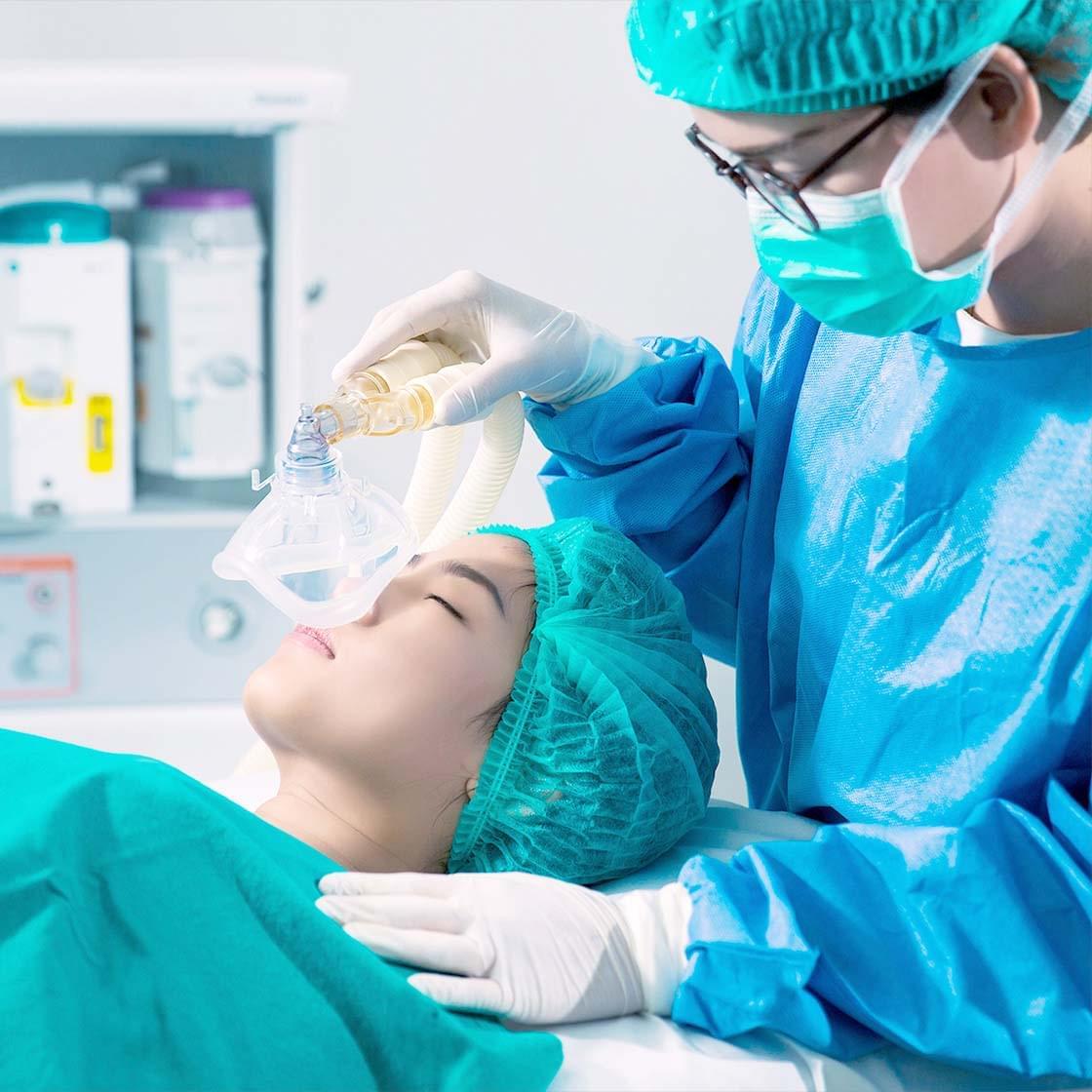
We continue to bring innovative new products to improve the quality-of-life (QOL) of patients. As a specialty pharmaceutical company developing anesthetics and antiseptics/disinfectants, we aim to provide full line-up of products in the fields of perioperative and infection control.